Surely this only works if the pasta is already in a metallic container made from some other metal than aluminum.
Surely this doesn’t work if pasta is in a plastic container.
Indeed:
edit
A “lasagna cell” is accidentally produced when salty moist food such as lasagna or sauerkraut is stored in a steel baking pan and is covered with aluminium foil. After a few hours the foil develops small holes where it touches the lasagna, and the food surface becomes covered with small spots composed of corroded aluminium
Alluminium oxide or microplastics, what’s your pick?
Aluminum oxide. Pretty damn inert.
I once electroplated some Spanish rice by making it in a cast iron pan and covering it tightly with aluminum foil.
Because I was in college I ate the parts that weren’t silver. It tasted normal.
Apparently, the product of the reaction is an aluminium salt and does not harm the food.
(Although the original source would not load for me)
We can turn this into some gourmet shit
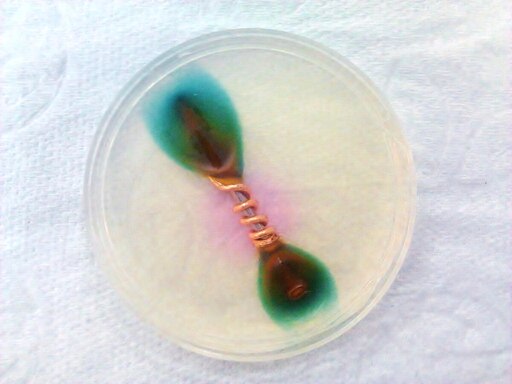
You can turn a lemon into a battery with some copper and zinc stuck right into the juicy part.
But are they combustible so I can burn life’s house down with them?
I mean… If you have enough lemons and sodium bicarbonate to generate a large enough exothermic reaction between the citric acid in the lemons and the baking soda, maybe? But it generates such a small amount of heat when making one of those kindergarten volcano science experiments so you’d probably need a lot before it was hot enough to combust, if it even got hotter with more materials reacting.
Aperture might have ideas I don’t, though. Ask Cave Johnson.
Il Dcell